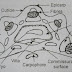
FILTRATION OF BULK SOLUTION
Process Of Filtration ! Filtration is the process of separating suspended solid matter from a liquid, by causing the latter to pass through the pores of a membrane, called a filter.
While preparing tea, a filter or a sieve is used to separate tea leaves from the water.
Filter sieve is called as filtering media, the tea leaves collected on filter mesh is called as filter cake and the solution passes from filter media and collected on collecting vessel is known as filtrate.
BULK SOLUTION- The solution present in bulk quantity for the filtration process.
FILTER MEDIA- It is the medium which is used for the filtration from which solution is passed or solid undissolved particles are retained on it.
It may be Paper,membrane, sintered glass Filter media or sintered filter candle.
The
v Filtration of the bulk solution is done by transferring the solution to the pressure vessel of any size like 50-100 L.
v Close the pressure vessel and attach the
silicon pipe to it.
v Open the tap of the filter assembly having
the two openings as shown in fig.
v To top end attach the pipe which is further
attached to top of pressure vessel
v And attach the bottom end of filtering
assembly to the top of another collecting vessel or pressure vessel.
v Attach another silicon tube to 1st
pressure vessel having solution to be filtered.
v Now Apply nitrogen gas which provide
pressure in vessel and solution from the vessel comes out from vessel to filter
assembly and filtration takes place
v Filtrate is collecting to collecting
vessel.
v Solid or undissolved particles are
retained on filter membrane or filter paper .